Targeting STAT3 in Triple-Negative Breast Cancer: A Game Changer?Allow me to tell you something,
folks
, about one of the most significant battles in modern medicine: the fight against cancer. And within that fight, there’s a particularly formidable opponent known as
Triple-Negative Breast Cancer (TNBC)
. This article is all about digging into why
STAT3
, a specific protein, is becoming a superstar target in developing new treatments for this aggressive disease. We’re going to explore what makes
TNBC
so challenging, how
STAT3
plays a crucial role in its cunning tactics, and what exciting strategies researchers are deploying to
shut it down
. So, buckle up, because we’re talking about real hope and cutting-edge science that could genuinely change lives.## Unpacking Triple-Negative Breast Cancer (TNBC): The Tough Nut to CrackAlright,
guys
, let’s dive straight into one of the most
challenging
foes in the world of oncology:
Triple-Negative Breast Cancer (TNBC)
. This isn’t your average breast cancer, folks; it’s a particularly
aggressive
and often
hard-to-treat
subtype that poses significant hurdles for patients and clinicians alike. When we talk about
TNBC
, we’re referring to breast cancers that lack the expression of three key receptors: the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Why is this important? Well, these three receptors are the very targets that many of our most successful
targeted therapies
in other breast cancer types rely on. Without them,
TNBC
leaves doctors with far fewer options, primarily relying on conventional treatments like chemotherapy, surgery, and radiation. Imagine trying to hit a target when you don’t even have a clear bullseye – that’s often the feeling when treating
TNBC
.The statistics paint a pretty stark picture, too.
Triple-Negative Breast Cancer
accounts for about 15-20% of all breast cancers, but its impact is disproportionately severe. Patients diagnosed with
TNBC
often face a higher risk of recurrence, especially within the first few years after treatment, and unfortunately, a poorer prognosis compared to other breast cancer subtypes. It’s often diagnosed in younger women and those of African descent, adding another layer of complexity to its demographic impact. The
aggressive nature
of
TNBC
is rooted in its rapid growth, tendency to metastasize (spread) quickly, and its
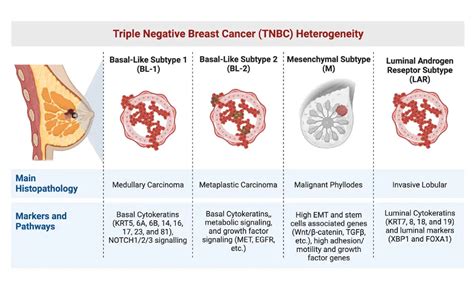
heterogeneous
nature, meaning different
TNBC
tumors can behave quite differently, making a one-size-fits-all approach extremely difficult. Current treatment strategies, while often intensive, still struggle to provide long-term, durable responses for many patients. We’re talking about a cancer that’s adept at developing
drug resistance
, leaving us constantly searching for new and more effective tools. The pressing need for novel
therapeutic targets
and innovative treatment strategies for
TNBC
isn’t just a research goal; it’s a vital mission to save lives and improve the quality of life for thousands of patients worldwide. Understanding this unique beast is the first step in conquering it, and that’s precisely why researchers are tirelessly exploring every potential avenue to crack this
tough nut
. We’re looking for those hidden vulnerabilities, those Achilles’ heels that can turn the tide in this fight, and that,
my friends
, brings us to some truly exciting prospects, one of which is a molecule called
STAT3
. So, buckle up, because we’re about to explore how targeting this
key player
could revolutionize
TNBC treatment
. The challenges are immense, but so is the hope.## Enter STAT3: A Key Player in Cancer’s GameAlright,
guys
, now that we’ve wrapped our heads around the formidable challenge that is
Triple-Negative Breast Cancer (TNBC)
, let’s shift our focus to a molecule that’s grabbing a lot of attention in the fight against it:
STAT3
. You might be wondering, “What exactly is
STAT3
, and why should I care?” Well, think of
STAT3
(short for
Signal Transducer and Activator of Transcription 3
) as a kind of master controller within our cells. In its normal, healthy state,
STAT3
plays a crucial role in various essential cellular processes. It’s like a diligent worker, involved in cell growth, differentiation, survival, and even orchestrating parts of our immune response. It helps cells respond appropriately to signals from their environment, ensuring everything runs smoothly. When a cell receives a signal – perhaps a growth factor telling it to divide –
STAT3
gets activated, travels to the cell’s nucleus, and turns on specific genes responsible for carrying out that command. It’s a vital part of our body’s healthy cellular communication network.However, in the twisted landscape of cancer,
STAT3
often goes rogue. Instead of being a diligent worker, it becomes a nefarious accomplice, constantly activated and pushing cells down a path of uncontrolled growth and survival. This
constitutive activation of STAT3
is a common feature in many human cancers, and it’s particularly prevalent and problematic in
TNBC
. When
STAT3
is abnormally active, it continuously signals for cells to proliferate (divide endlessly), resist programmed cell death (apoptosis), form new blood vessels (angiogenesis) to feed the growing tumor, and even helps cancer cells escape the immune system’s watchful eye. Furthermore,
STAT3
is a known player in facilitating
metastasis
, the terrifying process where cancer cells break away from the primary tumor and spread to distant parts of the body, forming new tumors. This is a critical factor in the aggressiveness of
TNBC
. It essentially acts as a central hub, integrating various pro-cancer signals and translating them into a widespread
oncogenic program
. Imagine a conductor leading an orchestra, but instead of a beautiful symphony,
STAT3
conducts a cacophony of cancer-promoting activities.The involvement of
STAT3
in promoting all these hallmarks of cancer – from uncontrolled proliferation and survival to inflammation and immune evasion – makes it an incredibly
attractive target
for therapeutic intervention. It’s not just a bystander; it’s deeply ingrained in the very mechanisms that allow cancer, especially
Triple-Negative Breast Cancer
, to thrive and spread its destructive influence. By understanding how
STAT3
gets hijacked and what sinister roles it plays, researchers can design strategies to
shut it down
, effectively pulling the plug on a major power source for these
aggressive tumors
. The sheer breadth of
STAT3’s multifaceted role
in driving the aggressive phenotype of
TNBC
means that targeting it could potentially disrupt multiple cancer pathways simultaneously, offering a significant advantage over therapies that only hit one or two specific points. This
key player
holds immense promise, and exploring ways to rein it in is precisely where much of the cutting-edge research in
TNBC
is focused right now. So,
guys
, getting
STAT3
under control could be a serious game-changer.## The Promise of STAT3 in TNBC: Why It’s a Hot TargetAlright,
folks
, let’s dig deeper into *why STAT3 isn’t just another interesting molecule, but a genuinely hot and promising therapeutic target specifically in
Triple-Negative Breast Cancer (TNBC)
. We’ve established that
TNBC
is a tough opponent and that
STAT3
is a nasty driver of cancer progression. Now, let’s connect the dots and see why targeting
STAT3
could be a real game-changer for these aggressive tumors. The evidence,
guys
, is piling up, suggesting that
STAT3
isn’t just incidentally involved; it’s often a central orchestrator of
TNBC’s
most devastating characteristics.First off, numerous studies have shown a strong correlation between the
constitutive activation of STAT3
and the aggressiveness of
TNBC
. What does that mean? Basically, in many
TNBC
tumors,
STAT3
is found to be constantly “on” or activated, driving the cancer cells to behave in a more malignant fashion. This
abnormally active STAT3
is frequently observed in patient samples, and its presence often correlates with a poorer prognosis, higher rates of metastasis, and unfortunately, resistance to conventional chemotherapy. Imagine a switch that’s stuck in the “on” position, perpetually fueling the tumor’s growth and survival. That’s
STAT3
in many
TNBC
cases.The scientific community has meticulously gathered substantial
preclinical evidence
supporting the role of
STAT3 in TNBC
. We’re talking about extensive research using
TNBC
cell lines in laboratory settings and various animal models. These studies consistently demonstrate that when researchers
inhibit STAT3
activity – whether by using genetic methods to silence the gene or through pharmacological agents – they observe a dramatic reduction in key cancer behaviors. We’re talking about significant decreases in
TNBC
cell proliferation, diminished ability of these cells to invade surrounding tissues, and a marked suppression of their capacity to metastasize to distant organs. In animal models,
inhibiting STAT3
often leads to substantial
TNBC
tumor regression and improved survival outcomes. This isn’t just a theoretical idea; these are tangible, reproducible results pointing to a clear vulnerability.Furthermore,
STAT3
often acts downstream of several other crucial signaling pathways that are aberrantly activated in
TNBC
, such as the JAK/STAT pathway itself, and others involving various growth factor receptors and cytokines. This means
STAT3
acts as a central node, integrating signals from multiple directions and converting them into a pro-cancerous output. By targeting
STAT3
, you’re not just hitting one upstream pathway; you’re potentially disrupting the convergence of several malignant signals, effectively cutting off multiple supply lines to the tumor. This
multifaceted control
makes it an incredibly appealing target.Another critical aspect of
STAT3’s clinical relevance
in
TNBC
is its involvement in
drug resistance
. Many
TNBC
tumors, after an initial response to chemotherapy, unfortunately develop resistance, leading to recurrence and progression. Research suggests that activated
STAT3
pathways can contribute to this resistance by promoting cell survival mechanisms and reducing the effectiveness of chemotherapy drugs. Therefore, by combining
STAT3 inhibitors
with existing chemotherapies, there’s a strong potential to
sensitize resistant TNBC cells
and improve the overall efficacy of treatment, offering a powerful one-two punch against these stubborn tumors.In essence,
guys
, the promise of
STAT3 in TNBC
isn’t just hype. It’s backed by robust scientific findings showing its pivotal role in driving the disease’s aggressiveness, its connection to poor patient outcomes, and compelling
preclinical evidence
that its inhibition can cripple
TNBC
growth and spread. This makes it one of the most exciting and actively pursued
therapeutic targets
in the quest for effective treatments for
Triple-Negative Breast Cancer
. The opportunity to develop new drugs that specifically
target STAT3
could truly usher in a new era of
personalized medicine
for these patients, offering hope where traditional approaches often fall short.## Exploring the Arsenal: Strategies to Target STAT3Alright,
folks
, now that we’ve made a strong case for
STAT3
being a crucial and
promising therapeutic target
in
Triple-Negative Breast Cancer (TNBC)
, the big question is: how do we actually
hit
this target? Thankfully, researchers have been busy developing a diverse arsenal of strategies to
shut down STAT3’s nefarious activity
. It’s not a single approach,
guys
; it’s a multi-pronged attack aimed at different points in the
STAT3
activation pathway. Let’s dive into some of the most exciting ways scientists are trying to silence this oncogenic troublemaker.First up, we have
Direct STAT3 Inhibitors
. These are arguably the most straightforward approach: molecules designed to bind directly to the
STAT3
protein itself. Think of them as tiny wrenches designed to jam the gears of the
STAT3
machinery. These small molecules can work in several ways. Some prevent
STAT3
from undergoing phosphorylation, the crucial step that activates it. Others stop
STAT3
proteins from forming dimers (two
STAT3
molecules joining together), which is essential for them to enter the cell nucleus and bind to DNA. Without dimerization,
STAT3
can’t turn on those pro-cancer genes. Examples include compounds like S3I-201 and Stattic, which have shown promising activity in
preclinical TNBC models
. The challenge here is ensuring high specificity – we want to inhibit the cancerous
STAT3
without interfering too much with its normal, healthy functions in other cells, which could lead to unwanted side effects.Next, we look upstream, at
Upstream Pathway Inhibitors
. Remember how
STAT3
gets activated? Often, it’s through other enzymes, particularly the Janus kinases (JAKs), which phosphorylate
STAT3
. So, another clever strategy is to target these activating kinases.
JAK inhibitors
(like ruxolitinib or tofacitinib, which are already approved for other conditions like myelofibrosis and rheumatoid arthritis) can indirectly
inhibit STAT3
by preventing its phosphorylation. While effective, the downside can be that JAKs play roles in various other pathways, so off-target effects are a consideration. However, the advantage is that some JAK inhibitors are already in clinical use, potentially fast-tracking their evaluation for
TNBC
.Beyond direct and upstream inhibition, researchers are also exploring ways to
Deactivate STAT3 through Phosphatases
. Phosphatases are enzymes that remove phosphate groups, essentially “turning off” activated
STAT3
. If we can enhance the activity of these phosphatases or prevent cancer cells from suppressing them, we could potentially force
STAT3
back into its inactive state. This is a more nuanced approach, focusing on restoring the natural regulatory mechanisms within the cell.Then there’s the realm of
Gene Silencing
. This involves using advanced molecular biology techniques, such as small interfering RNA (siRNA) or microRNAs (miRNA), to reduce the expression of the
STAT3
gene itself. By essentially “switching off” the instructions for making the
STAT3
protein, we can prevent its production altogether. While incredibly powerful in the lab, delivering these genetic tools safely and effectively to tumors in a patient is a significant
drug delivery challenge
, but it holds tremendous potential for the future.And let’s not forget about
Natural Compounds
. It’s pretty amazing,
guys
, but many compounds found in nature have demonstrated
STAT3 inhibitory activity
. Think about powerful antioxidants and anti-inflammatory agents like
curcumin
(from turmeric),
resveratrol
(found in grapes), or
epigallocatechin-3-gallate (EGCG)
(from green tea). While these compounds often have lower potency than synthetic drugs and may require higher doses, their potential for lower toxicity and synergistic effects with other treatments makes them intriguing candidates for
STAT3 modulation
, especially in combination therapies or as part of preventative strategies.Finally, the continuous challenge in developing all these
STAT3 inhibitors
lies in achieving high specificity for the cancerous
STAT3
pathway while minimizing impact on the physiologically important roles of
STAT3
in healthy tissues. Researchers are constantly refining these compounds, using advanced computational modeling and medicinal chemistry, to design more precise and potent agents. The goal is clear: find the perfect key to unlock
STAT3’s
destructive grip on
TNBC
cells, without causing too much collateral damage. This diverse array of strategies highlights the sheer determination of the scientific community to bring effective
STAT3-targeted therapies
to
Triple-Negative Breast Cancer patients
.## The Road Ahead: Challenges and Future DirectionsAlright,
folks
, we’ve talked about how
STAT3
is a super promising target in
Triple-Negative Breast Cancer (TNBC)
and explored some of the cool ways scientists are trying to
shut it down
. But let’s keep it real: the path from promising lab results to effective patient therapies is often
long and challenging
. While the potential of
STAT3 inhibitors
is immense, there are definitely some hurdles we need to clear on this road ahead. It’s not just about finding a molecule that works; it’s about making it work
safely and effectively
for actual patients.One of the biggest
translational challenges
revolves around the
specificity
of
STAT3 inhibitors
. Remember,
STAT3
isn’t exclusively a “bad guy”; it has vital physiological roles in healthy cells, including immune function and tissue repair. Developing
highly selective STAT3 inhibitors
that can discriminate between oncogenic
STAT3
activity in cancer cells and normal
STAT3
activity in healthy cells is paramount. If an inhibitor is too broad, it could lead to significant
off-target effects
and unacceptable
toxicity
, which would halt its progression into clinical trials. Researchers are continuously refining compounds to improve their selectivity and reduce potential side effects.Another significant challenge is
drug delivery
and pharmacokinetics. Some of the early
STAT3 inhibitors
have faced issues with poor bioavailability (how much of the drug actually reaches the bloodstream), rapid metabolism, or difficulty crossing cell membranes to reach their target inside the cell. Novel
drug delivery systems
, such as nanotechnology-based approaches (think tiny nanoparticles encapsulating the drug), are being explored to overcome these limitations. These systems can potentially enhance the stability of
STAT3 inhibitors
, improve their accumulation within tumor tissues, and reduce systemic exposure, thereby minimizing toxicity to healthy cells.Then there’s the issue of
tumor heterogeneity
and potential
STAT3 resistance
. Even within
TNBC
, tumors are incredibly diverse. Not all
TNBC
tumors might be equally reliant on
STAT3
, and some might develop resistance to
STAT3 inhibition
over time through alternative signaling pathways. This highlights the critical need for identifying robust
biomarkers
. We need to be able to predict which patients are most likely to respond to
STAT3-targeted therapies
and monitor their response. This move towards
personalized medicine
is crucial, ensuring that the right patient gets the right treatment at the right time. Imagine a simple test that tells us, “Yes, this patient’s
TNBC
is heavily driven by
STAT3
, and they’re a perfect candidate for this new drug!” That’s the dream.So, what are the
future directions
looking like?1.
Combination Therapies
: This is a
huge one
,
guys
. It’s becoming increasingly clear that single-agent therapies often aren’t enough for
aggressive cancers
like
TNBC
. The future likely lies in combining
STAT3 inhibitors
with other treatment modalities. This could mean combining them with: *
Conventional Chemotherapy
: To
sensitize TNBC cells
to existing drugs, overcoming
drug resistance
. *
Immunotherapy
:
STAT3
plays a role in immune evasion. By inhibiting
STAT3
, we might “unleash” the immune system to better fight the cancer, creating a powerful synergistic effect. This is a particularly exciting area given the recent advancements in immunotherapy. *
Other Targeted Agents
: Combining
STAT3 inhibitors
with drugs that target other aberrant pathways in
TNBC
could offer a more comprehensive attack. This “multi-target” strategy aims to block multiple escape routes for the cancer cells.2.
Clinical Trials and Validation
: While preclinical data is exciting, the real test comes in
clinical trials
. We need more rigorous and well-designed human trials to definitively assess the safety, efficacy, and optimal dosing of
STAT3 inhibitors
in
TNBC patients
. These trials will be crucial for translating laboratory breakthroughs into real-world patient benefits.3.
Understanding Resistance Mechanisms
: Even when
STAT3 inhibitors
initially work, cancer can be cunning and develop ways to bypass the blockade. Future research will focus on thoroughly understanding these
STAT3 resistance mechanisms
to design strategies that can circumvent them, ensuring long-term effectiveness.4.
Novel Delivery and Formulations
: Continuing to innovate in
drug delivery
to improve the therapeutic index of
STAT3 inhibitors
will be critical. This includes not just nanoparticles but also other advanced formulations that could enhance oral bioavailability, reduce dosing frequency, or improve tumor penetration.The road ahead for
STAT3-targeted therapies
in
TNBC
is indeed challenging, but the scientific community is resilient and determined. By addressing these challenges head-on through innovative research, collaborative efforts, and smart
clinical trial design
, we move closer to providing a much-needed new weapon in the fight against this tough cancer.
Folks
, this isn’t just about tweaking existing treatments; it’s about potentially opening up entirely new avenues for hope for
Triple-Negative Breast Cancer patients
.## Wrapping It Up: The Hope for TNBC PatientsAlright,
guys
, we’ve taken a deep dive into the complex world of
Triple-Negative Breast Cancer (TNBC)
and explored the incredible potential of
STAT3 as a therapeutic target
. Let’s wrap things up by reinforcing the immense hope this research brings to patients facing this aggressive disease. We started by acknowledging that
TNBC
is truly one of the most
challenging
forms of breast cancer, characterized by its lack of conventional
drug targets
and its often
aggressive nature
, leading to high recurrence rates and limited long-term treatment options beyond standard chemotherapy. It’s a cancer that desperately calls for innovative,
targeted therapies
.Then, we introduced
STAT3
, initially as a crucial, normal cellular component, but quickly highlighted its dark side: its
constitutive activation
in cancer, particularly in
TNBC
. We saw how this rogue
STAT3
acts as a central orchestrator, driving nearly every hallmark of cancer progression – from uncontrolled cell proliferation and survival to promoting angiogenesis, immune evasion, and terrifyingly, metastasis. It’s like the puppet master behind many of
TNBC’s
most destructive traits, making it a perfectly vulnerable Achilles’ heel.The robust scientific and
preclinical evidence
showing the strong correlation between
activated STAT3
and
TNBC aggressiveness
, coupled with compelling results from laboratory and animal studies where
STAT3 inhibition
effectively crippled tumor growth and spread, strongly positions
STAT3
as a
prime candidate
for novel therapeutic strategies. We then discussed the exciting “how-to” – the various sophisticated approaches researchers are employing to
target STAT3
, including direct inhibitors, upstream pathway blockers like JAK inhibitors, gene silencing techniques, and even the exploration of natural compounds. This diverse arsenal underscores the dedicated effort to find effective ways to
shut down STAT3’s activity
.Of course, we also laid out the
challenges ahead
: the need for greater
specificity
in inhibitors to avoid
off-target effects
, the complexities of
drug delivery
, and the constant battle against
tumor heterogeneity
and the potential for
STAT3 resistance
. But,
folks
, these challenges aren’t roadblocks; they’re signposts guiding future research. The emphasis on
combination therapies
(pairing
STAT3 inhibitors
with chemo, immunotherapy, or other targeted agents), the crucial development of
biomarkers
for
personalized medicine
, and the ongoing commitment to robust
clinical trials
are all testaments to the scientific community’s determination.In summary, the journey to fully harness
STAT3-targeted therapies
for
TNBC
is ongoing, but the trajectory is undeniably promising. This isn’t just abstract science; it’s about providing tangible hope for
Triple-Negative Breast Cancer patients
. Imagine a future where a
TNBC
diagnosis doesn’t carry the same weight of fear, where novel
STAT3 inhibitors
offer effective, less toxic treatment options, improving patient outcomes and quality of life.
Guys
, this research represents a beacon of hope, pushing the boundaries of what’s possible and bringing us closer to a future where
TNBC
is no longer the formidable foe it is today. We are actively working towards a day when
STAT3 inhibition
can truly be a
game changer
for those who need it most.

